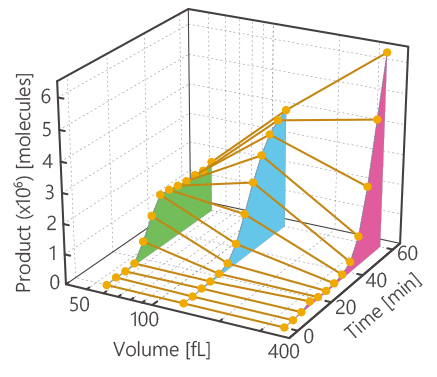
Volume dependence of protein synthesis
Compartment size dependency
The size and shape of living cells change depending on the cell cycle and in response to the external environment. In contrast, the cell maintains the copy number of the genome constant. Thus, in all cells, the protein synthesis takes place from a constant number of genes, while the reaction volume may vary. We speculated that, because the protein synthesis is one of the nonlinear reactions consisting of transcription, translation, and possibly multimer formation, its dynamics may be highly sensitive to the conditions including the reaction volume.
We investigated the effects of compartment volume on the protein synthesis, β-glucuronidase (GUS) or β-galactosidase (GAL) synthesis, triggered by a constant copy number of DNA in glass microchambers. GUS and GAL are tetrameric enzymes and their synthesis are a fourth- and a first-order reaction, respectively. A large variation in the time course for GUS synthesis was observed among the different chamber volumes, whereas it was not for GAL. This result indicates that the tetrameric GUS synthesis was accelerated in a smaller chamber. However, the product increase stopped earlier in the smaller chamber due to the more rapid complete hydrolysis of the substrate in the smaller chamber. We found that as a result of such circumstances, the GUS synthesis has an optimum compartment volume in which the quantity of the product is maximized.
Collective dynamics of chemical oscillators
Deformation of giant unilamellar vesicles

Protein synthesis from a single copy of DNA
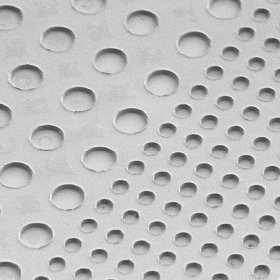
Volume dependence of protein synthesis
Droplet & Microchannel
(Paper in progress)

Impact sensor
(Ring!Ring!Project)